- Clinical Study
- Serum Transferrin Predicts New-Onset Type 2 Diabetes in Koreans: A 4-Year Retrospective Longitudinal Study
-
Jong Dai Kim, Dong-Mee Lim, Keun-Young Park, Se Eun Park, Eun Jung Rhee, Cheol-Young Park, Won-Young Lee, Ki Won Oh
-
Endocrinol Metab. 2020;35(3):610-617. Published online September 22, 2020
-
DOI: https://doi.org/10.3803/EnM.2020.721
-
-
4,395
View
-
98
Download
-
5
Web of Science
-
5
Crossref
-
 Abstract Abstract
 PDF PDF PubReader PubReader  ePub ePub
- Background
It is well known that high serum ferritin, a marker of iron storage, predicts incident type 2 diabetes. Limited information is available on the association between transferrin, another marker of iron metabolism, and type 2 diabetes. Thus, we investigated the association between transferrin and incident type 2 diabetes.
Methods
Total 31,717 participants (mean age, 40.4±7.2 years) in a health screening program in 2005 were assessed via cross-sectional analysis. We included 30,699 subjects who underwent medical check-up in 2005 and 2009 and did not have type 2 diabetes at baseline in this retrospective longitudinal analysis.
Results
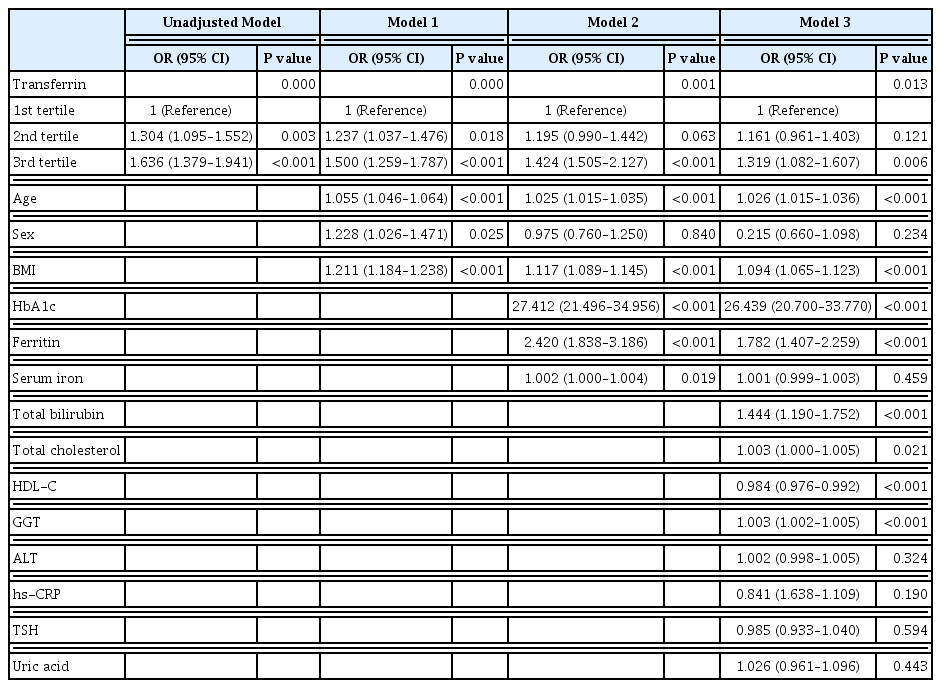
The serum transferrin level was higher in the type 2 diabetes group than in the non-type 2 diabetes group (58.32±7.74 μmol/L vs. 56.17±7.96 μmol/L, P<0.001). Transferrin correlated with fasting serum glucose and glycosylated hemoglobin in the correlational analysis (r=0.062, P<0.001 and r=0.077, P<0.001, respectively) after full adjustment for covariates. Transferrin was more closely related to homeostasis model assessment of insulin resistance than to homeostasis model assessment of β cell function (r=0.042, P<0.001 and r=–0.019, P=0.004, respectively) after full adjustment. Transferrin predicted incident type 2 diabetes in non-type 2 diabetic subjects in a multivariate linear regression analysis; the odds ratio (95% confidence interval [CI]) of the 3rd tertile compared to that in the 1st tertile of transferrin for incident diabetes was 1.319 (95% CI, 1.082 to 1.607) after full adjustment (P=0.006).
Conclusion
Transferrin is positively associated with incident type 2 diabetes in Koreans.
-
Citations
Citations to this article as recorded by  - Plasma proteome profiling reveals the therapeutic effects of the PPAR pan-agonist chiglitazar on insulin sensitivity, lipid metabolism, and inflammation in type 2 diabetes
Xingyue Wang, You Wang, Junjie Hou, Hongyang Liu, Rong Zeng, Xiangyu Li, Mei Han, Qingrun Li, Linong Ji, Desi Pan, Weiping Jia, Wen Zhong, Tao Xu
Scientific Reports.2024;[Epub] CrossRef - Plasma Proteomic Signature of Endometrial Cancer in Patients with Diabetes
Muhammad Mujammami, Mohamed Rafiullah, Khalid Akkour, Assim A. Alfadda, Afshan Masood, Salini Scaria Joy, Hani Alhalal, Maria Arafah, Eman Alshehri, Ibrahim O. Alanazi, Hicham Benabdelkamel
ACS Omega.2024; 9(4): 4721. CrossRef - Association between systemic iron status and β-cell function and insulin sensitivity in patients with newly diagnosed type 2 diabetes
Yao Qin, Yiting Huang, Yuxiao Li, Lu Qin, Qianying Wei, Xin Chen, Chuanhui Yang, Mei Zhang
Frontiers in Endocrinology.2023;[Epub] CrossRef - Association of Body Iron Metabolism with Type 2 Diabetes Mellitus in Chinese Women of Childbearing Age: Results from the China Adult Chronic Disease and Nutrition Surveillance (2015)
Jie Feng, Xiaoyun Shan, Lijuan Wang, Jiaxi Lu, Yang Cao, Lichen Yang
Nutrients.2023; 15(8): 1935. CrossRef - Serum Level of Ceruloplasmin, Angiotensin-Converting Enzyme and Transferrin as Markers of Severity in SARS-CoV-2 Infection in Patients with Type 2 Diabetes
Patricia-Andrada Reștea, Ștefan Țigan, Laura Grațiela Vicaș, Luminița Fritea, Eleonora Marian, Tunde Jurca, Annamaria Pallag, Iulius Liviu Mureșan, Corina Moisa, Otilia Micle, Mariana Eugenia Mureșan
Microbiology Research.2023; 14(4): 1670. CrossRef
- Clinical Study
- Effects of Short-Term Exenatide Treatment on Regional Fat Distribution, Glycated Hemoglobin Levels, and Aortic Pulse Wave Velocity of Obese Type 2 Diabetes Mellitus Patients
-
Ju-Young Hong, Keun-Young Park, Byung-Joon Kim, Won-Min Hwang, Dong-Ho Kim, Dong-Mee Lim
-
Endocrinol Metab. 2016;31(1):80-85. Published online March 16, 2016
-
DOI: https://doi.org/10.3803/EnM.2016.31.1.80
-
-
4,966
View
-
49
Download
-
26
Web of Science
-
22
Crossref
-
 Abstract Abstract
 PDF PDF PubReader PubReader
- Background
Most type 2 diabetes mellitus patients are obese and have obesity related vascular complications. Exenatide treatment is well known for both decreasing glycated hemoglobin levels and reduction in body weight. So, this study aimed to determine the effects of exenatide on body composition, glycated hemoglobin levels, and vascular stiffness in obese type 2 diabetes mellitus patients. MethodsFor 1 month, 32 obese type 2 diabetes mellitus patients were administered 5 µg of exenatide twice daily. The dosage was then increased to 10 µg. Patients' height, body weight, glycated hemoglobin levels, lipid profile, pulse wave velocity (PWV), body mass index, fat mass, and muscle mass were measured by using Inbody at baseline and after 3 months of treatment. ResultsAfter 3 months of treatment, glycated hemoglobin levels decreased significantly (P=0.007). Triglyceride, total cholesterol, and low density lipoprotein levels decreased, while aspartate aminotransferase and alanine aminotransferase levels were no change. Body weight, and fat mass decreased significantly (P=0.002 and P=0.001, respectively), while interestingly, muscle mass did not decrease (P=0.289). In addition to, Waist-to-hip ratio and aortic PWV decreased significantly (P=0.006 and P=0.001, respectively). ConclusionEffects of short term exenatide use in obese type 2 diabetes mellitus with cardiometabolic high risk patients not only reduced body weight without muscle mass loss, body fat mass, and glycated hemoglobin levels but also improved aortic PWV in accordance with waist to hip ratio.
-
Citations
Citations to this article as recorded by  - Adipose tissue inflammation linked to obesity: A review of current understanding, therapies and relevance of phyto-therapeutics
Christiana Eleojo Aruwa, Saheed Sabiu
Heliyon.2024; 10(1): e23114. CrossRef - Separate and combined effects of empagliflozin and semaglutide on vascular function: A 32‐week randomized trial
Liv Vernstrøm, Søren Gullaksen, Steffen S. Sørensen, Kristian L. Funck, Esben Laugesen, Per L. Poulsen
Diabetes, Obesity and Metabolism.2024; 26(5): 1624. CrossRef - Diabetic Sarcopenia. A proposed muscle screening protocol in people with diabetes
Daniel de Luis Román, Juana Carretero Gómez, José Manuel García-Almeida, Fernando Garrachón Vallo, German Guzmán Rolo, Juan José López Gómez, Francisco José Tarazona-Santabalbina, Alejandro Sanz-Paris
Reviews in Endocrine and Metabolic Disorders.2024;[Epub] CrossRef - The Current Landscape of Pharmacotherapies for Sarcopenia
Gulistan Bahat, Serdar Ozkok
Drugs & Aging.2024; 41(2): 83. CrossRef - Vascular Aging: Assessment and Intervention
Ao Li, Jinhua Yan, Ya Zhao, Zhenping Yu, Shane Tian, Abdul Haseeb Khan, Yuanzheng Zhu, Andong Wu, Cuntai Zhang, Xiao-Li Tian
Clinical Interventions in Aging.2023; Volume 18: 1373. CrossRef - The Effect of Additional Treatment with Empagliflozin or Semaglutide on Endothelial Function and Arterial Stiffness in Subjects with Type 1 Diabetes Mellitus—ENDIS Study
Maja Preložnik Navodnik, Andrej Janež, Ivan Žuran
Pharmaceutics.2023; 15(7): 1945. CrossRef - Sarcopenia as a Little-Recognized Comorbidity of Type II Diabetes Mellitus: A Review of the Diagnosis and Treatment
Christian Salom Vendrell, Elisa García Tercero, Juan Bautista Moro Hernández, Bernardo Abel Cedeno-Veloz
Nutrients.2023; 15(19): 4149. CrossRef - Oral semaglutide improves body composition and preserves lean mass in patients with type 2 diabetes: a 26-week prospective real-life study
Sara Volpe, Giuseppe Lisco, Margherita Fanelli, Davide Racaniello, Valentina Colaianni, Valentina Lavarra, Domenico Triggiani, Lucilla Crudele, Vincenzo Triggiani, Carlo Sabbà, Giovanni De Pergola, Giuseppina Piazzolla
Frontiers in Endocrinology.2023;[Epub] CrossRef - GLP1 Receptor Agonists—Effects beyond Obesity and Diabetes
Sydney S. Wilbon, Mikhail G. Kolonin
Cells.2023; 13(1): 65. CrossRef - The Effectiveness of GLP-1 Receptor Agonist Semaglutide on Body Composition in Elderly Obese Diabetic Patients: A Pilot Study
Yoshinori Ozeki, Takayuki Masaki, Akari Kamata, Shotaro Miyamoto, Yuichi Yoshida, Mitsuhiro Okamoto, Koro Gotoh, Hirotaka Shibata
Medicines.2022; 9(9): 47. CrossRef - Clinical Recommendations to Manage Gastrointestinal Adverse Events in Patients Treated with Glp-1 Receptor Agonists: A Multidisciplinary Expert Consensus
Juan J. Gorgojo-Martínez, Pedro Mezquita-Raya, Juana Carretero-Gómez, Almudena Castro, Ana Cebrián-Cuenca, Alejandra de Torres-Sánchez, María Dolores García-de-Lucas, Julio Núñez, Juan Carlos Obaya, María José Soler, José Luis Górriz, Miguel Ángel Rubio-H
Journal of Clinical Medicine.2022; 12(1): 145. CrossRef - The Impact of Glucose-Lowering Drugs on Sarcopenia in Type 2 Diabetes: Current Evidence and Underlying Mechanisms
Elena Massimino, Anna Izzo, Gabriele Riccardi, Giuseppe Della Pepa
Cells.2021; 10(8): 1958. CrossRef - Anti‐diabetic drugs and sarcopenia: emerging links, mechanistic insights, and clinical implications
Xueli Zhang, Yi Zhao, Shuobing Chen, Hua Shao
Journal of Cachexia, Sarcopenia and Muscle.2021; 12(6): 1368. CrossRef - Effect of glycemic control on markers of subclinical atherosclerosis in patients with type 2 diabetes mellitus: A review
Sofia Antoniou, Katerina K K Naka, Marios Papadakis, Aris Bechlioulis, Agathocles Tsatsoulis, Lampros K Michalis, Stelios Tigas
World Journal of Diabetes.2021; 12(11): 1856. CrossRef - A Review of the Effects of Glucagon-Like Peptide-1 Receptor Agonists and Sodium-Glucose Cotransporter 2 Inhibitors on Lean Body Mass in Humans
Jack Alistair Sargeant, Joseph Henson, James Adam King, Thomas Yates, Kamlesh Khunti, Melanie Jane Davies
Endocrinology and Metabolism.2019; 34(3): 247. CrossRef - The effect of dulaglutide on body composition in type 2 diabetes mellitus patients on hemodialysis
Takahiro Yajima, Kumiko Yajima, Hiroshi Takahashi, Keigo Yasuda
Journal of Diabetes and its Complications.2018; 32(8): 759. CrossRef - Effects of Newer Antidiabetic Drugs on Endothelial Function and Arterial Stiffness: A Systematic Review and Meta-Analysis
Konstantinos Batzias, Alexios S. Antonopoulos, Evangelos Oikonomou, Gerasimos Siasos, Evanthia Bletsa, Panagiota K. Stampouloglou, Chara-Vasiliki Mistakidi, Marina Noutsou, Niki Katsiki, Periklis Karopoulos, Georgios Charalambous, Anastasia Thanopoulou, N
Journal of Diabetes Research.2018; 2018: 1. CrossRef - Regulation of visceral and epicardial adipose tissue for preventing cardiovascular injuries associated to obesity and diabetes
N. González, Z. Moreno-Villegas, A. González-Bris, J. Egido, Ó. Lorenzo
Cardiovascular Diabetology.2017;[Epub] CrossRef - Articles inEndocrinology and Metabolismin 2016
Won-Young Lee
Endocrinology and Metabolism.2017; 32(1): 62. CrossRef - Difference in protective effects of GIP and GLP-1 on endothelial cells according to cyclic adenosine monophosphate response
Dong-Mee Lim, Keun-Young Park, Won-Min Hwang, Ju-Young Kim, Byung-Joon Kim
Experimental and Therapeutic Medicine.2017; 13(5): 2558. CrossRef - Treatment Strategy for Type 2 Diabetes with Obesity: Focus on Glucagon-like Peptide-1 Receptor Agonists
Qiuhe Ji
Clinical Therapeutics.2017; 39(6): 1244. CrossRef - Differential Role of Adipose Tissues in Obesity and Related Metabolic and Vascular Complications
Almudena Gómez-Hernández, Nuria Beneit, Sabela Díaz-Castroverde, Óscar Escribano
International Journal of Endocrinology.2016; 2016: 1. CrossRef
- Endocrine Research
- Omega-3 Polyunsaturated Fatty Acids May Attenuate Streptozotocin-Induced Pancreatic β-Cell Death via Autophagy Activation in Fat1 Transgenic Mice
-
Won-Min Hwang, Dong-Ho Bak, Dong Ho Kim, Ju Young Hong, Seung-Yun Han, Keun-Young Park, Kyu Lim, Dong-Mee Lim, Jae Gu Kang
-
Endocrinol Metab. 2015;30(4):569-575. Published online December 31, 2015
-
DOI: https://doi.org/10.3803/EnM.2015.30.4.569
-
-
4,074
View
-
43
Download
-
17
Web of Science
-
19
Crossref
-
 Abstract Abstract
 PDF PDF PubReader PubReader
- Background
Inflammatory factors and β-cell dysfunction due to high-fat diets aggravate chronic diseases and their complications. However, omega-3 dietary fats have anti-inflammatory effects, and the involvement of autophagy in the etiology of diabetes has been reported. Therefore, we examined the protective effects of autophagy on diabetes using fat-1 transgenic mice with omega-3 self-synthesis capability. MethodsStreptozotocin (STZ) administration induced β-cell dysfunction in mice; blood glucose levels and water consumption were subsequently measured. Using hematoxylin and eosin (H&E) and Masson's trichrome staining, we quantitatively assessed STZ-induced changes in the number, mass, and fibrosis of pancreatic islets in fat-1 and control mice. We identified the microtubule-associated protein 1A/1B light chain 3-immunoreactive puncta in β-cells and quantified p62 levels in the pancreas of fat-1 and control mice. ResultsSTZ-induced diabetic phenotypes, including hyperglycemia and polydipsia, were attenuated in fat-1 mice. Histological determination using H&E and Masson's trichrome staining revealed the protective effects of the fat-1 expression on cell death and the scarring of pancreatic islets after STZ injection. In the β-cells of control mice, autophagy was abruptly activated after STZ treatment. Basal autophagy levels were elevated in fat-1 mice β-cells, and this persisted after STZ treatment. Together with autophagosome detection, these results revealed that n-3 polyunsaturated fatty acid (PUFA) enrichment might partly prevent the STZ-related pancreatic islet damage by upregulating the basal activity of autophagy and improving autophagic flux disturbance. ConclusionFat-1 transgenic mice with a n-3 PUFA self-synthesis capability exert protective effects against STZ-induced β-cell death by activating autophagy in β-cells.
-
Citations
Citations to this article as recorded by  - Mitochondrial Dysfunction and Mitophagy in Type 2 Diabetes: Pathophysiology and Therapeutic Targets
Nadezda Apostolova, Teresa Vezza, Jordi Muntane, Milagros Rocha, Víctor M. Víctor
Antioxidants & Redox Signaling.2023; 39(4-6): 278. CrossRef - β Cell and Autophagy: What Do We Know?
Hamid-Reza Mohammadi-Motlagh, Mona Sadeghalvad, Niloofar Yavari, Rosita Primavera, Setareh Soltani, Shashank Chetty, Abantika Ganguly, Shobha Regmi, Tina Fløyel, Simranjeet Kaur, Aashiq H. Mirza, Avnesh S. Thakor, Flemming Pociot, Reza Yarani
Biomolecules.2023; 13(4): 649. CrossRef - The ameliorating effects of mesenchymal stem cells compared to α‐tocopherol on apoptosis and autophagy in streptozotocin‐induced diabetic rats: Implication of PI3K/Akt signaling pathway and entero‐insular axis
Heba A. Mubarak, Manal M. Kamal, Yossra Mahmoud, Fatma S. Abd‐Elsamea, Eman Abdelbary, Marwa G. Gamea, Reham I. El‐Mahdy
Journal of Cellular Biochemistry.2023; 124(11): 1705. CrossRef - Nutraceuticals as Modulators of Autophagy: Relevance in Parkinson’s Disease
Michał Rakowski, Szymon Porębski, Agnieszka Grzelak
International Journal of Molecular Sciences.2022; 23(7): 3625. CrossRef - High n-3 fatty acids counteract hyperglycemia-induced insulin resistance in fat-1 mice via pre-adipocyte NLRP3 inflammasome inhibition
Qingyao Yu, Tiantian Wang, Feng Wang, Yong Yang, Canxia He, Wenge Yang, JinJie Zhang, Zuquan Zou
Food & Function.2021; 12(1): 230. CrossRef - Metabolic and Metabolomic Insights Regarding the Omega-3 PUFAs Intake in Type 1 Diabetes Mellitus
Carmen Purdel, Anca Ungurianu, Denisa Margina
Frontiers in Molecular Biosciences.2021;[Epub] CrossRef - Effects of the linoleic acid/docosahexaenoic acid ratio and concentration inducing autophagy in Raw264.7 cells against Staphylococcus aureus
Li-Ying Xu, Min Mu, Man-Li Wang, Jin-Cheng Liu, Yuan-Jie Zhou, Jing Wu, Bing-You Jiang, Ming-Gong Chen, Dong Hu, Xing-Rong Tao
Journal of Clinical Biochemistry and Nutrition.2020; 67(2): 146. CrossRef - Free fatty acid receptor 3 differentially contributes to β-cell compensation under high-fat diet and streptozotocin stress
Medha Priyadarshini, Connor Cole, Gautham Oroskar, Anton E. Ludvik, Barton Wicksteed, Congcong He, Brian T. Layden
American Journal of Physiology-Regulatory, Integrative and Comparative Physiology.2020; 318(4): R691. CrossRef - Mitochondria and T2D: Role of Autophagy, ER Stress, and Inflammasome
Milagros Rocha, Nadezda Apostolova, Ruben Diaz-Rua, Jordi Muntane, Victor M. Victor
Trends in Endocrinology & Metabolism.2020; 31(10): 725. CrossRef - Omega-3 Polyunsaturated Fatty Acids Prevent Toxoplasma gondii Infection by Inducing Autophagy via AMPK Activation
Choi, Lee, Lee, Park, Lee, Shin, Cha, Lee, Lim, Yuk
Nutrients.2019; 11(9): 2137. CrossRef - Survivin regulated by autophagy mediates hyperglycemia-induced vascular endothelial cell dysfunction
Yu-Xue Xu, Caoxin Huang, Minyi Liu, Ningning Chen, Wenting Chen, Chen Yang, Yan Zhao, Xuejun Li, Junguo Duan, Suhuan Liu, Shuyu Yang
Experimental Cell Research.2018; 364(2): 152. CrossRef - Autophagy in Metabolic Age-Related Human Diseases
Manon Moulis, Cecile Vindis
Cells.2018; 7(10): 149. CrossRef - A comparison of the anti-diabetic potential of d-ribose-l-cysteine with insulin, and oral hypoglycaemic agents on pregnant rats
Abraham A.A. Osinubi, Leke Jacob Medubi, Edidiong N. Akang, Lawal K. Sodiq, Titilola A. Samuel, Taiwo Kusemiju, James Osolu, Danladi Madu, Olufemi Fasanmade
Toxicology Reports.2018; 5: 832. CrossRef - β-Cell Autophagy in Diabetes Pathogenesis
Michelle R Marasco, Amelia K Linnemann
Endocrinology.2018; 159(5): 2127. CrossRef - Endogenous synthesis of n-3 polyunsaturated fatty acids in fat-1 transgenic mice ameliorates streptozocin-induced diabetic nephropathy
Yuan-Ming Zhang, Xiao-Hong Zhang, Pan Zhu, Rong-Hui Tan, Jin-Shun Zhao, Feng Wang, Jin-Jie Zhang, Wang Yan, Yang Xi, Jian-Bo Wan, Jing-Xuan Kang, Zu-Quan Zou, Shi-Zhong Bu
Journal of Functional Foods.2018; 45: 427. CrossRef - PHLPP: a putative cellular target during insulin resistance and type 2 diabetes
Alpana Mathur, Vivek Kumar Pandey, Poonam Kakkar
Journal of Endocrinology.2017; 233(3): R185. CrossRef - Coenzyme Q10 ameliorates cerebral ischemia reperfusion injury in hyperglycemic rats
Cui-Jie Lu, Yong-Zhen Guo, Yang Zhang, Lan Yang, Yue Chang, Jing-Wen Zhang, Li Jing, Jian-Zhong Zhang
Pathology - Research and Practice.2017; 213(9): 1191. CrossRef - Changes in pancreatic histology, insulin secretion and oxidative status in diabetic rats following treatment with Ficus deltoidea and vitexin
Samsulrizal Nurdiana, Yong Meng Goh, Hafandi Ahmad, Sulaiman Md Dom, Nur Syimal’ain Azmi, Noor Syaffinaz Noor Mohamad Zin, Mahdi Ebrahimi
BMC Complementary and Alternative Medicine.2017;[Epub] CrossRef - Comparative analysis of the efficacy of omega-3 fatty acids for hypertriglyceridaemia management in Korea
H.-S. Kim, H. Kim, Y. J. Jeong, S. J. Yang, S. J. Baik, H. Lee, S.-H. Lee, J. H. Cho, I.-Y. Choi, H. W. Yim, K.-H. Yoon
Journal of Clinical Pharmacy and Therapeutics.2016; 41(5): 508. CrossRef
|